The M120 Risk Score: Improving Identification of Children at High Risk of Developing Clinical Type 1 Diabetes
Type1Screen’s Prof John Wentworth and colleagues have released a new paper. The purpose was to assess if the M120 could more accurately predict a child’s risk of developing type 1 diabetes as well as help determine responsiveness to immunotherapy (in this case Teplizumab).
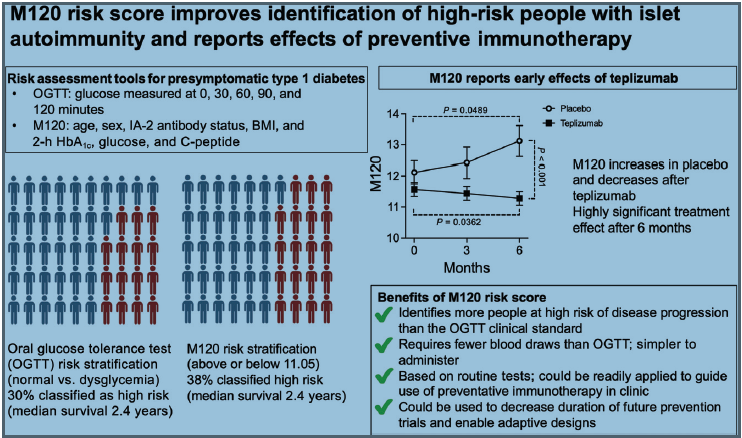
What is M120? M120 is a calculation using age, BMI, and islet antibody (IA-2A) status alongside HbA1c, glucose, and C-peptide measured from a single blood sample collected 120 min after oral glucose. Its requirement for a single blood draw improves acceptability and feasibility, particularly for young children.
ARTICLE HIGHLIGHTS
Why did we undertake this study?
We aimed to determine if the M120 risk score could enrich for disease risk beyond glucose measures alone and report short-term treatment
effects.
What is the specific question(s) we wanted to answer?
Is M120 superior to the oral glucose tolerance test for risk stratification, and can it identify early effects of teplizumab?
What did we find?
M120 identified 26% more children with multiple autoantibodies at risk of disease progression, including many with normoglycemia. Within 6
months, M120 decreased after teplizumab treatment and increased after placebo treatment to reveal a highly significant treatment effect.
What are the implications of our findings?
M120 could be used as a primary outcome measure in future prevention trials to rapidly assess new therapies, enable adaptive designs, and guide
treatment decisions in the clinic.
Access the full article at: https://doi.org/10.2337/dc24-2794


